Spirogyra
NIGLYTIN
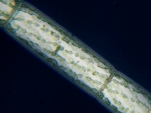
Brightfiel, GG
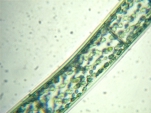
Darkfield, GG
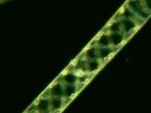
Phase contrast, GG
Mounting with NIGLYTIN
Production of NIGLYTIN
As NIGLYTIN is not available any more, the original recipe for this mounting medium is unknown. The recipe given here is that of "new NIGLYTIN", but this medium seems to possess similar qualities.
Mix 7 grams of gelatine with 60 ml of distilled water, wait some hours, then heat the liquid in a water bath until a clear solution has formed. This solution is mixed with 50 ml of glycerol (80%), 2 grams of "nigrosine water-soluble" and 0.6 gram of phenol. Stir for some time at 70 oC (water bath), then filter at the same temperature in an oven. The product has the same qualities as glycerol gelatine.
NOTE: Use only gelatine free of insoluble impurities. This must be checked in a preliminary test by microscopic inspection.
Suitable objects
Blue-green algae (cyanobacteria), filamentous green algae, all kinds of planktonic blue-green algae.
Fixation and pretreatment
The objects are treated with formol (1 ml of formaldehyde 40% per 10 ml sample), ar prefeably with PFEIFFER´s mixture (remove the water and cover with PFEIFFER). After 2 days remove the chemical fixative, then cover with 50 ml of diluted glycerol (5 grams per 100 ml distilled water), transfer the material into an open jar with a flat bottom and let the water evaporate slowly in a dust-free place. Finally add concentrated glycerol and store the objects in small specimen glasses.
PFEIFFER´s mixture
| Formaldehyde 40% | 100 ml | |
| Vinegar made from wood | 100 ml | |
| Methanol (not ethanol !) | 100 ml |
Since "vinegar made from wood" is no longer available, use 100 ml of vinegar and add 3 ml of creosote to the mixture (300 ml).
How to use NIGLYTIN
Transfer a very small amount of the objects on an object slide, remove most of the glycerol and mix with a very small drop of liquid NIGLYTIN (melt it at about 60 oC on a water bath). After mixing the two media very thoroughly with needles cover the mixture with a round coverslip. Press this coverslip gently. The NIGLYTIN should become somewhat translucent (bluish black, not "pitch black"). It is of utmost importance that the objects touch the inner surfaces of the coverslip and the object slide as well. Sometimes little pieces of metal (e.g. nuts) put on the coverslide are useful. Allow the NIGLYTIN to dry completely, remove NIGLYTIN which has oozed out, cleanse with alcohol and protect the prepared slide with a ring of varnish.
Sometimes it is better to transfer the objects directly after the fixation on an object slide and to cover them with diluted glycerol. When the water has evaporated they are mounted with NGILYTIN then. This method helps to avoids flaws.
Note: The objects must touch the coverslip as well as the object slide, otherwise they will be masked by black NIGLYTIN. Therefore filamentous algae should form only one layer (see above)!
Some examples
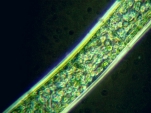
If the method is applied properly, the algae stand out brightly against a dark background.
The real significance of NIGLYTIN is to make slime fully visible. Even experienced microscopists are often dumbfounded when they suddenly realize that certain algae (especially colonies of cyanobacteria) show a large brightly lit halo of mucus that was earlier completely invisible before! In this respect the method is even superior to the phase contrast method.
 |
 |
 |
 |
|
|
Spirogyra |
Spirogyra Brightfiel, GG |
Spirogyra Darkfield, GG |
Spirogyra Phase contrast, GG |
The NIGLYTIN preparation clearly shows a halo of mucus which remains invisible in brightfield and darkfield. Phase contrast gives an extremely bright edge but ultimately displays only the cell wall.
Using NIGLYTIN requires some experience: If the slide shows bright flaws (which cannot be completely avoided), the glycerol was not properly mixed with the NIGLYTIN. If we see "black algae on a black background", the NIGLYTIN layer is too thick - between the slide and the coverslip only one layer of objects is allowed, the objects touching the slide and the coverslip as well.